Honest, paywall-free news is rare. Please support our boldly independent journalism with a donation of any size.
In recent months, anti-trans activists have targeted gender-affirming care, asserting that the evidence supporting it isn’t “high quality” according to the Grades of Recommendation, Assessment, Development, and Evaluation (GRADE) system’s standards — a system that places a premium on randomized clinical trials. However, scientific experts and federal court judges have found the use of the term “high quality evidence” to be highly misleading. In fact, roughly 90% of all medical care lacks “high quality evidence” based on this system, but there are not similar calls to ban those medications and procedures. This discrepancy largely stems from the fact that randomized clinical trials are often impractical or unethical for many medications and conditions. Now, researchers in Melbourne, Australia, recently conducted the first randomized clinical trial to study the impact of gender-affirming care on transgender individuals. Their findings were stunning: gender-affirming care led to an reduction of suicidality in 55% of the treatment group receiving hormones, compared with just 5% in the control group.
Researchers in Melbourne, Australia, studied 64 transmasculine individuals who sought testosterone. Participants were randomly split into a treatment group and a control group. The treatment group received their gender-affirming care within a week, while the control group waited three months. Both groups were evaluated for suicidality, dysphoria, and depression. Three months later, they were assessed again. The findings were striking: Suicidality scores halved for the treatment group, but remained unchanged for the control group. Depression scores also decreased by half in the treatment group. Additionally, gender dysphoria scores diminished for those receiving treatment.
See the results here:
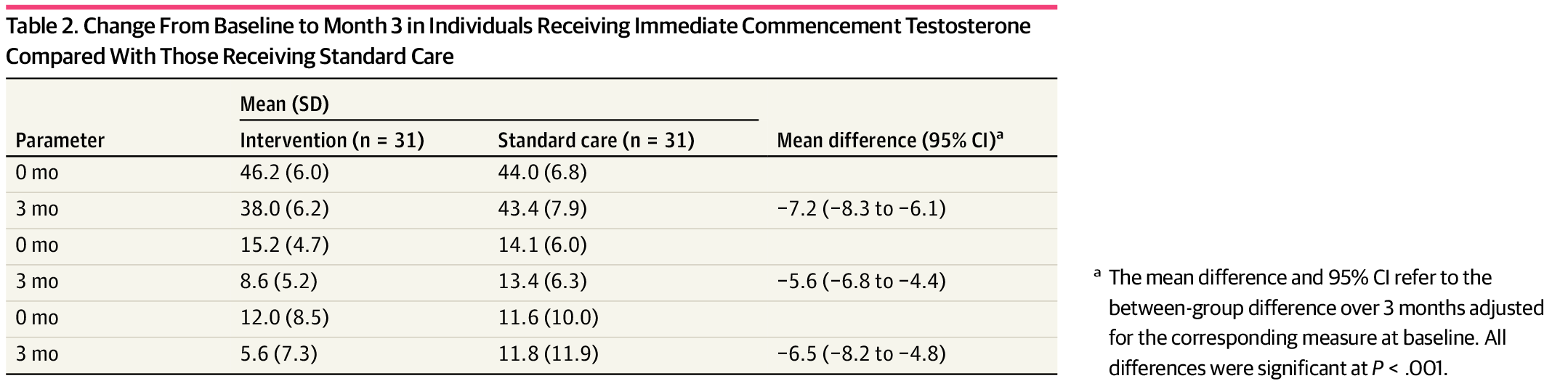
These scores are well in line with a huge body of observational research showing a reduction in suicidality and depression among trans individuals receiving care. Another study with a much longer follow-up period found a 73% reduction in suicidality. A similar study found a 40% reduction in actual suicide attempts over the previous year. Cornell University has compiled over 50 studies that show evidence for the benefits of gender affirming care. Due to this large and growing body of evidence, a recent article in The Lancet declared that gender affirming care should be treated as preventative care for transgender people.
This study is the first of its kind. Conducting randomized controlled trials for gender-affirming care, similar to many conventional medical treatments, is unethical — a fact that the researchers in this study acknowledge. Withholding medication that has years of clinical backing and a plethora of studies would likely face rejection from most institutional review boards. The challenge isn’t unique to gender-affirming care. Roughly 90% of medical treatments lack “high-quality evidence” under the GRADE system’s standards. Yet, this doesn’t imply that these treatments are “untested, experimental, or questionable.” The GRADE system doesn’t block individualized medical care supported by a rich collection of observational studies, purposefully so. This list of medical care backed by similar-level evidence includes antidepressants, radiation therapy, the majority of neonatal and pediatric medications, the practice of not giving aspirin for child fevers, mammograms, gallbladder surgeries, and more — nobody calls for banning these medical treatments.
In order to get around the ethical problems of withholding care, the researchers studied only adults, and opted for a shorter follow-up period. The researchers point out that by doing this, the control group would not be disadvantaged for very long, and that such a wait period would be deemed acceptable for transgender people who want to contribute to such important research. This means that such a trial could be conducted ethically.
See their statement on this decision:
This short period was designed for participant acceptability and feasibility so that transgender and gender-diverse participants would not be disadvantaged by waiting longer than standard care waiting times of 3 months for an initial consultation. Second, participants were not blinded to their intervention group. Therefore, it is possible that the effect of testosterone or patient knowledge of treatment has been evaluated. However, randomization of participants to no treatment or placebo over longer follow-up is unethical, particularly given preexisting barriers to accessing GAHT.
The improvement in depression and suicidality underscores the substantial benefits of gender-affirming care. The reduction scores align with the much longer observational studies, which typically report a 40-73% decrease in suicidality. While this study alone doesn’t confirm long-term advantages, when viewed alongside the extensive clinical experiences of physicians and numerous other studies indicating long-term benefits of such care, it establishes the importance of this care.
Recently, anti-trans activists have pointed to the absence of RCTs as grounds to prohibit the care, neglecting to mention that the evidence for gender-affirming care aligns with that of most other medical treatments with similar absences. With the release of this study, this argument is no longer tenable. Critics may shift to critiquing the study’s brief follow-up duration, sidestepping the fact that extended observational studies have consistently validated these outcomes for transgender individuals. Some will likely advocate for “treatment groups” that withhold hormone therapy from transgender individuals for years, despite the damage doing so would cause. At the very least, this study highlights the critical importance of immediate access to hormone therapy for transgender individuals and solidifies its use as compelling evidence in future decisions for clinicians.
Note: This piece was republished with permission from Erin In The Morning.
Media that fights fascism
Truthout is funded almost entirely by readers — that’s why we can speak truth to power and cut against the mainstream narrative. But independent journalists at Truthout face mounting political repression under Trump.
We rely on your support to survive McCarthyist censorship. Please make a tax-deductible one-time or monthly donation.
