
Joseph Borzelleca has been evaluating the safety of food additives longer than pretty much anyone else in the business. The 84-year-old toxicologist, who credits his career to Italian parents who taught him to love food, has helped companies bring hundreds of new ingredients to market.
“Food to me was always very important,” said Borzelleca, a long-time professor at Virginia Commonwealth University, who has been reviewing the safety of food additives since the 1960s. “I had an interest in food, not just from a nutritional perspective but from a historical and safety” perspective.
A Center for Public Integrity analysis of publicly available data found that Borzelleca is the most active of a small group of scientists – including several with ties to Big Tobacco – that the food industry turns to over and over again to determine whether additives can be deemed “generally recognized as safe,” or GRAS, and avoid a rigorous pre-market government safety review.
Of the 379 panels convened to review the safety of new ingredients in the last 17 years, the Center for Public Integrity found, three-quarters included at least one of these 10 scientists. But none has even come close to serving on as many as Borzelleca, who has appeared on 41 percent of them.
Despite his decades of experience and praise heaped upon him by colleagues – one called him a “wonder” – critics of the GRAS system say Borzelleca is emblematic of a system that is rife with conflicts of interest. If scientists depend on the food industry for income, they may be less likely to contest the safety of ingredients companies hope to market, critics say.
“These are standing panels of industry hired guns,” said Laura MacCleery, an attorney for the Center for Science in the Public Interest. “It is funding bias on steroids.”
Borzelleca and many of his colleagues who work with the food industry have done similar work for another well-known industry: Big Tobacco.
The Center for Public Integrity found that at least four of the top 10 GRAS panel experts, including Borzelleca, had also served as scientific consultants for cigarette makers.
Final Word
The expert panels that review a new food additive to determine if it’s “generally recognized as safe” have great power because they can have the final word on that ingredient and its use. Once the group deems a new additive GRAS, it can go into an array of foods that end up on supermarket shelves, with no notice to or review by the US Food and Drug Administration.
That gives food companies an incentive to turn to experts they believe will look kindly upon their ingredients, and gives scientists incentive to do so, critics say.
“If I know that my paycheck is coming from a specific source, and I’ve been doing that for years and years, and that is what feeds me and my family, it becomes really difficult for me to be totally independent of the hand that is feeding me,” said Erik Olson, senior strategic director for health and food at the Natural Resources Defense Council.
Many scientific consultants dispute accusations that they are conflicted, arguing instead that they are the most qualified and most experienced scientists for the job.
“If you’re good at something, of course you’re going to be in demand,” Borzelleca said, adding that he focuses solely on safety and never considers companies’ marketing plans. “I know GRAS pretty well. It’s not a boast. It’s a statement of fact.”
A Small World
A 1958 law allows companies to market ingredients without oversight by the US Food and Drug Administration if they can establish that their ingredients are “generally recognized as safe” for specific uses. In other words, companies using the so-called GRAS process must demonstrate that there is a consensus among scientific experts that their ingredients are safe.
To do so, they usually convene a panel of scientists to review articles and opinions of authoritative bodies like the National Academy of Sciences to determine if an ingredient is safe for a particular use. Of 562 publicly available GRAS determinations voluntarily submitted to the FDA since 1998, a Center for Public Integrity analysis found that companies used such panels two-thirds of the time.
These panels, typically composed of three members, are meant to represent the scientific community at large. And they are particularly useful for establishing scientific consensus “when an individual published study raises safety questions” about an ingredient, according to FDA guidance.
“As long as you adhere to science-based review,” said John Thomas, a scientific consultant, “then I don’t think there’s a better peer-reviewed process in place.”
The world of GRAS panelists is a small one. A Center for Public Integrity analysis found that the top 10 most frequently hired panelists have each sat on two dozen or more panels.
Borzelleca has participated in 156 panels in the last 17 years – almost three times as many as either of the next two most popular panelists: Thomas, an adjunct professor at the Indiana University School of Medicine, and Michael Pariza, a professor emeritus and former director of the University of Wisconsin’s industry-funded Food Research Institute.
Thomas and Pariza have each served on more than 50 panels that evaluated the safety of ingredients.
Often times, the same team of experts serve on panels together. Borzelleca and Pariza, for example, have teamed up on more than 40 panels.
“It’s not a large universe of people,” said Steve Morris of the Government Accountability Office, which published a report in 2010 that cited financial conflicts of interest in the GRAS system as a concern. “The fact that there’s … repetition and there’s familiarity, that could potentially breed a conflict.”
The Center for Public Integrity’s analysis likely captures only a fraction of all expert panels convened to establish the GRAS status of additives. That’s because companies can make safety evaluations in secret, without ever telling the FDA. So it’s unclear in those cases whether an expert panel made the determinations.
Companies are allowed to hire a single consultant to sign off on safety determinations or rely on the judgment of their own experts – and did so about a third of the time, according to the Center for Public Integrity’s analysis.
In 2013, a Pew Charitable Trusts review of GRAS assessments concluded that “financial conflicts of interest were ubiquitous” in the system.
“The lack of independent review in GRAS determinations raises concerns about the integrity of the process and whether it ensures the safety of the food supply,” Pew researchers concluded.
“Sterling Reputations, Impeccable Credentials”
Industry consultants dispute such criticisms.
“There’s a reason you keep going back to the same people and that’s because these are people with sterling reputations, impeccable credentials and they know what they’re doing,” said James Heimbach, who has convened GRAS panels for decades. “If you need a quadruple bypass, do you want to go to somebody who’s already done a bunch of them or do you want to go to someone who’s never done one before?”
Indeed, most experts have decades of experience making GRAS determinations and maintain affiliations at universities. Panelists typically have extensive scientific backgrounds, often at the doctorate level, with years of experience in toxicology, pharmacology, organic chemistry and biochemistry.
Consultants interviewed for this story stressed that they would never sign off on an additive if it put public safety or their own professional reputations at risk.
George Burdock, the president and founder of Burdock Group Consultants, noted in an email that the experts who conduct GRAS assessments all have families who eat food.
“Would you approve anything you knew was not safe, knowing that your family might consume a product containing the ingredient?” he asked.
Some scientists say they’ve participated in panels that have been disbanded because the experts could not agree that the ingredient was GRAS for its intended use.
“If they don’t meet the criteria, they’re rejected,” said consultant A. Wallace Hayes, who has appeared on nine GRAS panels.
There are no public records documenting how often this happens, however.
Some additives that companies hope to market never even reach a panel. A couple of consultants told the Center for Public Integrity that they often conduct preliminary safety evaluations with companies to review ingredients before the company invests in a more comprehensive review with a panel of experts.
 The GRAS Associates website explains the philosophy behind its butterfly logo. (Image: https://www.gras-associates.com) Robert McQuate, a former FDA regulator who co-founded the consulting firm GRAS Associates, says he sometimes flags safety problems during early screenings. He recalls doing so twice in the past few months.
The GRAS Associates website explains the philosophy behind its butterfly logo. (Image: https://www.gras-associates.com) Robert McQuate, a former FDA regulator who co-founded the consulting firm GRAS Associates, says he sometimes flags safety problems during early screenings. He recalls doing so twice in the past few months.
“We’re going to do a rigorous safety evaluation,” McQuate said. “There’s no guarantee you’re going to get what you want.”
Tobacco Ties
The Center for Public Integrity identified 10 GRAS panelists who have in the past had ties to the tobacco industry, including two who were once full-time employees of big tobacco companies, according to a review of tobacco industry documents archived by the University of California, San Francisco.
In interviews, some of these panelists said that their work for the tobacco industry was limited to evaluating the safety of cigarette additives or newly developed cigarette products that tobacco companies thought would be less dangerous. They stressed that they did not defend the safety of cigarettes in general.
Of the top 10 most frequently hired GRAS panelists, four have worked as consultants for tobacco companies.
Borzelleca’s work for the tobacco industry dates back at least to the early 1980s.
An RJ Reynolds memo from 1984 notes that Borzelleca “has been secured by the tobacco industry to represent our position” during discussions with the Department of Health and Human Services about cigarette additives.
Two years later, the company envisioned Borzelleca as its “main spokesman” if a list of cigarette additives submitted to HHS were leaked to the press and “there is sustained and intense media coverage of ingredients issues,” according to a confidential memo regarding the tobacco industry’s “public relations strategy.”
“My advice to the tobacco industry was that the GRAS substances they were using are safe when ingested,” Borzelleca wrote in an emailed response to Center for Public Integrity questions, “but I could not comment on their effects when they were subjected to the high temperatures of a lighted cigarette, a position that I still have.”
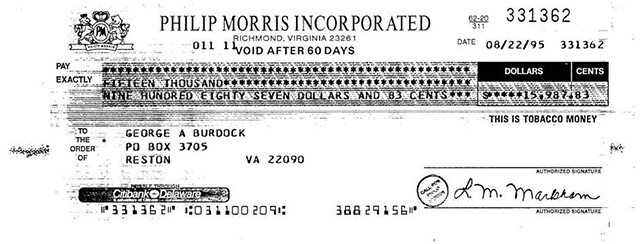 A check from Philip Morris Incorporated to George Burdock in 1995. (Image: Legacy Tobacco Documents Library/ University of California, San Francisco)
A check from Philip Morris Incorporated to George Burdock in 1995. (Image: Legacy Tobacco Documents Library/ University of California, San Francisco)
In 1995, Philip Morris hired Borzelleca for $2,000 a day for consulting services “relative to all aspects of tobacco processing and cigarette manufacture and to hold himself available to serve as a member of the Philip Morris USA Scientific Advisory Board.”
Borzelleca said he became a member of Philip Morris’ Scientific Advisory Board “to advise PM on a less hazardous cigarette,” which he added was “erroneously referred to as a ‘safer cigarette.’ “
However, he said he began donating his fees from his tobacco work to charity after officials from the United Nations Food and Agriculture Organization, with which he was involved as an expert, expressed concerns about his ties to Philip Morris.
Borzelleca said he has not consulted with tobacco companies for at least three years.
“This Is Tobacco Money”
For a while, Borzelleca’s colleagues on the Philip Morris Scientific Advisory Board included Pariza, Steve Taylor and William Waddell – scientists who are among the top 15 most contracted experts for safety assessments of ingredients added to food. Pariza and Taylor were also retained separately as consultants for the company.
Taylor told the Center for Public Integrity that he consulted with the company on safety-related issues for some of the ingredients in tobacco products, but he declined to work on the safety of tobacco itself because he “considered it a dangerous product.” Pariza did not respond to requests for comment.
Some GRAS panelists did more than just consult for tobacco companies.
A. Wallace Hayes served as an executive for RJR Nabisco, where he worked on both tobacco and food safety issues. A 1990 performance record said one of Hayes’ “objectives” was to “increase our knowledge base regarding the role of nicotine/cotinine in smoking enjoyment/satisfaction.”
“I only worked on RJ Reynolds’ attempts to develop a less hazardous, safe cigarette,” Hayes said. “I did not work on the traditional cigarette.”
Edward Carmines, who has participated in at least three GRAS panels, spent 13 years working as a scientist for Philip Morris. When contacted by phone and asked to discuss his work as a GRAS panelist, Carmines declined to comment and hung up.
Burdock Group Consultants is among the top five consulting firms hired by ingredient manufacturers for GRAS assessments. Burdock, the firm’s president, has participated in 10 expert panels since 1998.
While Burdock’s detailed résumé notes his extensive experience assessing the safety of food and dietary supplements for companies and trade associations, nowhere does it mention that Philip Morris routinely hired him as a scientific consultant in the 1990s and early 2000s.
On Aug. 22, 1995, Burdock received a check for nearly $16,000 from Philip Morris for his services. Underneath the amount, the check states: “This is tobacco money.”
When asked about his work for tobacco companies, Burdock replied in an email, “I cannot discuss any work I may have done with a client.”
Hayes, the former RJ Reynolds executive, said he “can see where people would have a problem” with former tobacco employees and consultants reviewing the safety of food additives. “But it’s all in the eyes of the beholder.”
“The Good, The Bad and The Ugly
Experts who serve on GRAS panels might think that they’re acting independently and making scientifically sound decisions, but that’s unlikely the case, says Sunita Sah, a Georgetown University business ethics professor who specializes in conflicts of interest.
Sah says many studies have shown that people’s judgment can be influenced by financial conflicts, even those involving as little as $5.
“For these scientists who are getting paid over and over again to do this, it’s very hard to remain totally impartial,” said Sah, who gave a presentation to industry consultants during a 2013 workshop about potential conflicts of interest in the GRAS system. “For something as important as what goes into somebody’s food, you want to eliminate those conflicts of interest.”
In 2010, the GAO recommended that the FDA “develop a strategy to minimize the potential for conflicts of interest in companies’ GRAS determinations.”
Five years later, the FDA says it’s still working on it.
Some industry consultants say they are open to the FDA creating guidelines to address concerns about conflicts of interest. But they worry that reforms could result in losing the most experienced expert panelists.
“It’s totally appropriate to investigate and refine and improve the processes with conflicts of interest,” McQuate said, “but let’s not throw the baby out with the bathwater.”
Borzelleca, for his part, says he would prefer to see the FDA weed out unqualified experts with limited experience reviewing GRAS additives. He worries that some scientists “gloss over things” when conducting safety reviews.
“You do get the good, the bad and the ugly,” Borzelleca said, adding that he has refused to serve on panels with scientists who had never conducted a GRAS assessment before. “We need to have some policing here.”
Join us in defending the truth before it’s too late
The future of independent journalism is uncertain, and the consequences of losing it are too grave to ignore. To ensure Truthout remains safe, strong, and free, we need to raise $43,000 in the next 6 days. Every dollar raised goes directly toward the costs of producing news you can trust.
Please give what you can — because by supporting us with a tax-deductible donation, you’re not just preserving a source of news, you’re helping to safeguard what’s left of our democracy.